

|
|
 |
|
There is no synthetic gemstone on the market that has had a greater impact on the jewelry industry than cubic zirconia. "CZ" as it is sometimes referred to in the industry, came on the market around 1978. And the prices at first were quite high when compared to current price levels. A one carat "Russian" cubic zirconia was selling for around US$20.00+ per carat wholesale. And business was good. Before we look into the gemological aspects of cubic zirconia, we should first look at the history. Because there is quite a bit of history involved. WHY? The first question that usually comes to mind is why cubic zirconia was first made. And just as synthetic diamonds and synthetic corundum had their beginning in industrial applications, so did cubic zirconia. The first cubic zirconia was made in Russia, for the purpose of laser technology. It seems the Russians did not have enough natural rubies that were required at the time to generate laser beams. So they set about to find a synthetic material that would have the properties of ruby. Their development: Cubic Zirconia. Not that CZ is particularly close to a ruby gemologically, but optically it served the purpose for the Russian laser technology. And of course, someone noticed that the new material would be very nice in jewelry since it also looked a lot like a diamond. So the ball got rolling to start producing CZ as jewelry items. Which leads us to where we are today. |
|
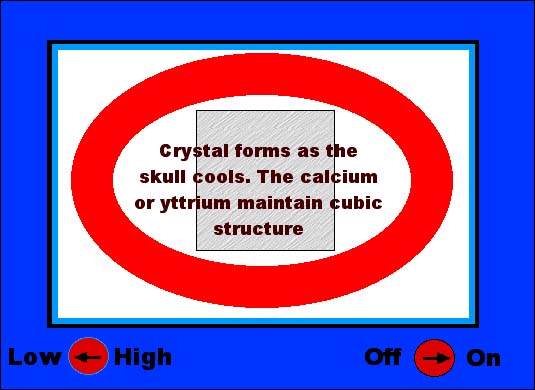
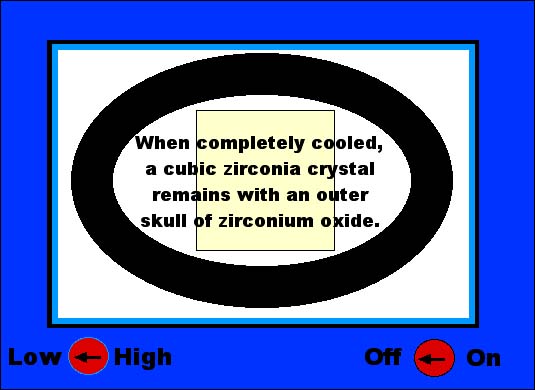
The gemology of cubic zirconia... Cubic zirconia is sort of an oxymoron...that meaning that it is really a contradiction of terms. Mainly because zirconium is not only a metal, but it crystallizes in the tetragonal crystal system in the form of the gemstone called zircon. However, zircon is a zirconium silicate, while a cubic zirconia is a zirconium oxide. The importance of this is that by substituting the oxygen for the silicon, the zirconium metal can be forced to crystallize in the cubic crystal system. Now, when I say forced to crystallize I mean exactly that. Zirconium metal with silicon (and in nature) will always form in the tetragonal crystal system. (See The 7 Crystal Systems here on YourGemologist.com). But by adding just a touch of either the elements calcium or yttrium, these elements have the ability to stabilize the zirconium metal to form crystals with the oxygen that have the properties of the cubic crystal system. The real importance of this fact is that diamonds are also cubic in crystal formation. So that any real imitation of diamond will have to also be cubic or they will never look quite right. As in the case of the synthetic moissanite. (see Synthetic Moissanite here on YourGemologist.com) The problem with making a cubic zirconia, however, is the amount of heat it takes to get the zirconium oxide mixture to melt. The temperature is so high that there is no crucible or container that will withstand that much heat. Not even platinum will take the heat required to melt zirconium oxide. Which is why we did not see cubic zirconium on the market until the late 1970' to early 1980's. Why? Because we needed a microwave oven. Just like the one that may be in your kitchen. What the microwave did is to give a method of synthetic crystal growth called a skull melt. This means that the material itself makes its own crucible. Since a microwave oven heats from the inside out, this allows for the interior of the substance to become very, very hot, while the outer layer stays cools and forms a crust that holds the molten interior. This is how the skull is formed and how we get the term skull melting. Let's take a graphic look at how the skull melting method works to form cubic zirconia crystals... As seen below, a powdered mixture of zirconium oxide is mixed into a microwave oven. Along with the ZrO2 is some calcium or yttrium as a stabilizer. |
 |
|
|
 |
|
|
 |
|
|
 |
|
|
 |
|
|
  |
|
|
 |
|
|
 |
|
There are many colors that can be produced with cubic zirconia. I have not attempted to obtain photographs of them because they are so wide spread and so cheap to buy. And just about any material you find called pink ice or something like that will be a colored version of cubic zirconia. Gemological Properties: There are a number of ways to identify a cubic zirconia. But before we go there let's list the properties according to the Gemmologist's Compendium by Webster: Chemical: Zirconium Oxide (ZrO2) Crystal System: Cubic Refractive Index: 2.18 average. Can run a bit higher or lower but will be single refractive Specific Gravity: 5.50 to 6.00 (again can vary) Hardness: 8.5 to 9 Imitates almost any colorless gemstone. Mostly used to imitate a diamond. Identification: Since it is over the reading ability of most refractometers, normal RI is not available unless you have a Jemeter or other reflectance meter. Most often identified by the rounding of facet junctions since CZ will never take the fine edge polish of a diamond at the facet junctions. Also, something called read through, where CZ will allow light to travel through at certain angles that diamonds will not. Most notable separation can be done using thermal conductivity meters which are widely used for ID purposes to separate diamond from CZ. Use of these is not recommended by YourGemologist, however, since synthetic moissanite will give false readings on these meters. And gemologists who rely solely on the thermal meter will be open to problems with the synthetic moissanite stones. So a properly trained gemologist will be able to identify CZ by its physical properties as the most accurate means. This will take experience and practice with known stones, hwoever, to master the ID process. |
|
That pretty well sums up the cubic zirconia for now. I do have more to add later. Specifically....I have a specimen of cubic zirconia that was made back in 1980 where an effort was made to put inclusions inside the faceted product. They did not look very real but they did fool a lot of untrained jewelers. And could do so today should one come across the counter in most stores. Also, I am working to photograph the read through effect that might be of service to you all. I will add those as soon as I can get the proper images to post. Until then, I hope you enjoyed this discussion on the history, production, and properties of cubic zirconia. Until next time, Robert |
|
© Copyright 2004 All Rights Reserved. Please read the fine print below: The information contained in this website is offered free of charge to anyone wishing to learn more about gemology. The information may be downloaded by any student, consumer, or jeweler for your own personal study and use. None of this site can be downloaded for posting on another website or server for any reason. It will be a violation of the copyright for anyone to copy, duplicate, distribute, and/or re-print this material in any format or any medium without written permission. Nor can anyone post this information on a for-profit website without written permission. That will ruin it for everyone and cause the entire site to be erased and canceled. Please honor this copyright for the good of everyone else. Robert James FGA, GG.....YourGemologist |
|
|