
 Synthetic Alexandrite
Synthetic AlexandriteConsumer Information
What color is it?: In this view under 10x in incandescent light you can see the strong red light produced. The green color under fluorescent is difficult to photograph in digital but I will be adding this to this page as soon as I can get the lighting problem worked out.
What is the story behind this gemstone?: For many years...in fact since the late 1800's, a type of imitation alexandrite has been on the market. This was actually a synthetic corundum which was treated with vanadium to give it its characteristic color change effect. Unfortunately, the color change was not a true imitation of the natural alexandrite and was fairly easy to identify by a good gemologist. Since 1973, however, true synthetic alexandrite has been produced by both the flux-melt and pulled crystal methods. And while the characteristic inclusions should give it away to a qualified gemologist, more than one retail jeweler has been fooled by this excellent synthetic alexandrite.
|
Source: Most synthetic alexandrites are produced in Japan and Russia, although other sources are available and will certainly be added here if I have missed listing them.
Chemical: As a synthetic alexandrite the chemical composition is identical: BeAl2O4, an oxide of beryllium and aluminum
Formation: In flux-melt crystals or larger sizes by the pulled method.
Crystal System: Orthorhombic (same as natural)
Unusual Properties: Distinct color change and cat's eyes have been produced that closely rival the natural.
Gemological Information and Identification
Since the synthetic alexandrite will possess all of the properties of the natural, most gemological tests will not help in the separation. Therefore, separation from natural alexandrite best done by internal inclusions as shown below:
Primary Test: Inclusions
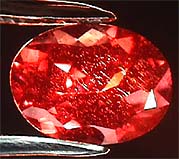
 A
pulled synthetic alexandrite of 2.50cts showing classic rain inclusions
A
pulled synthetic alexandrite of 2.50cts showing classic rain inclusions
 At
left the synthetic alexandrite above under 30x.
At
left the synthetic alexandrite above under 30x.
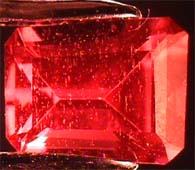 A
synthetic alexandrite of 2.10 carats under 10x.
A
synthetic alexandrite of 2.10 carats under 10x.
 The
above synthetic alexandrite under 30x.
The
above synthetic alexandrite under 30x.
Secondary Test: Ultraviolet Reactions
 Synthetic alexandrites under Long Wave
Ultraviolet
Synthetic alexandrites under Long Wave
Ultraviolet
 Synthetic Alexandrites under Short Wave
Ultraviolet
Synthetic Alexandrites under Short Wave
Ultraviolet
Please note: due to higher iron content that reduces reactions to ultraviolet in natural alexandrites (much the same as with ruby) the reactions will be an indication but not diagnostic. If possible you should have a natural alexandrite as a control stone if you are going to rely on UV to test of natural -v- synthetic for anything more than an indication or confirming test.
I would have some photographs of some natural alexandrites to compare but, after all, this is a free site and these gemstones are sort of expensive. Perhaps someone will let YourGemologist.com borrow a nice natural alexandrite to photograph for a future date.
Third Test: Is it too good to be true...
Natural alexandrites are very, very expensive. If you have a gem dealer come into your store and offers you alexandrites that seem at a very good price...keep your wits about you. There are no new fantastic finds of natural alexandrite reported anywhere in the world as of this writing. And any alexandrites of good color change and fairly good clarity will be very expensive. So be wary. Use your common sense as a test. It could be worth far more than all of the above information.
 Incandescent
Light..............Fluorescent Light
Incandescent
Light..............Fluorescent Light  Synthetic
Alexandrite reacts far more like its natural counterpart than
previous imitation gemstones such as vanadium treated corundum.
Here is an example of the excellent color change of a rough cross
section of a synthetic alexandrite crystal.
Synthetic
Alexandrite reacts far more like its natural counterpart than
previous imitation gemstones such as vanadium treated corundum.
Here is an example of the excellent color change of a rough cross
section of a synthetic alexandrite crystal.